
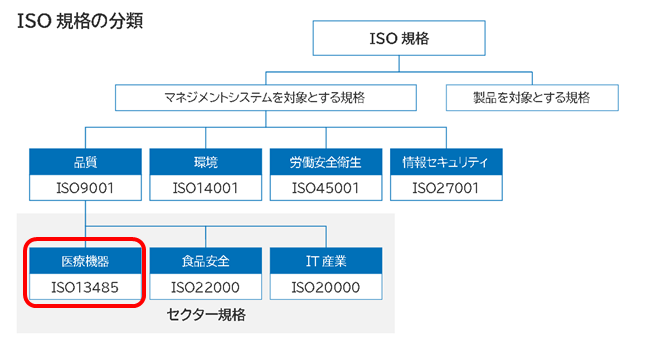
At Sze Pak Tech Co., Ltd. we are preparing to apply for ISO13485 medical device quality management system certification to further enhance our quality management and increase our credibility in the medical device industry.
What is ISO13485? ISO13485 is an international standard for quality management systems for the design, development, production, installation, and servicing of medical devices. Obtaining this certification indicates that our products and services meet international quality standards.
Purpose of Certification Application
· Quality Improvement: Further enhance product quality and provide safe and reliable medical devices to our customers.
· Increase Credibility: Enhance credibility in the medical device industry and create new business opportunities.
· International Market Compliance: By complying with international standards, promote entry into overseas markets and enhance global competitiveness.
Current Progress Currently, our company is reviewing and improving internal processes and strengthening employee training to obtain ISO13485 certification. These measures further reinforce our quality management system and prepare us for successful certification.
Future Outlook By obtaining ISO13485 certification, we will further enhance our quality management capabilities and establish leadership in the medical device industry. We will continue to provide high-quality products and services to meet the trust of our customers.
We sincerely hope for your continued support and trust.

Wuxi Service Hotline:+(86) 510-80299288
Fax :+(86) 510-80299282
Add:No.3,Xinrong Road,Xinwu District ,Wuxi,Jiangsu,China.
Company profile
Honorary qualification
Corporate culture
Automobile
OA equipment
Medical care
Smart home
Exhibition
Company news
Mold processing equipment
Forming equipment
Measuring Equipment
